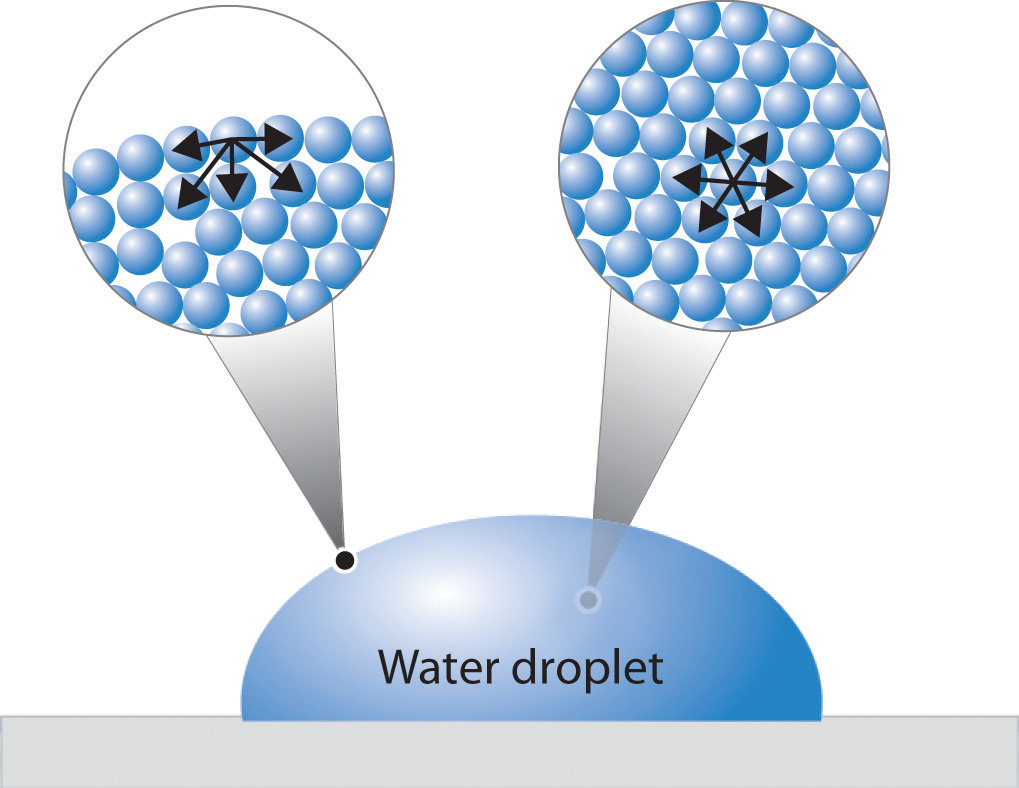
Measuring Surface Tension Of Water With A Penny . You can fit many more drops of water on top of a penny. In order to do this, you will determine the number of. attraction of the water molecules holds the surface of the water together until the amount of water is too great and spills over the. Fill a glass, cup, or small bowl with tap water. in this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. Fill the medicine dropper with water. water has very unique properties, and this experiment puts them to the test!. place your penny on a flat, level surface that can get a little wet, like a kitchen counter. 15k views 14 years ago. Water, soapy water, or rubbing alcohol. surface tension is given by the equation s = (ρhga/2) where s is the surface. in this portion of the lab you will determine which liquid has the highest surface tension:
from chemwiki.ucdavis.edu
surface tension is given by the equation s = (ρhga/2) where s is the surface. Fill the medicine dropper with water. in this portion of the lab you will determine which liquid has the highest surface tension: Water, soapy water, or rubbing alcohol. In order to do this, you will determine the number of. Fill a glass, cup, or small bowl with tap water. place your penny on a flat, level surface that can get a little wet, like a kitchen counter. in this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. water has very unique properties, and this experiment puts them to the test!. You can fit many more drops of water on top of a penny.
11.3 Unique Properties of Liquids Chemwiki
Measuring Surface Tension Of Water With A Penny water has very unique properties, and this experiment puts them to the test!. in this portion of the lab you will determine which liquid has the highest surface tension: place your penny on a flat, level surface that can get a little wet, like a kitchen counter. 15k views 14 years ago. You can fit many more drops of water on top of a penny. water has very unique properties, and this experiment puts them to the test!. attraction of the water molecules holds the surface of the water together until the amount of water is too great and spills over the. Fill the medicine dropper with water. surface tension is given by the equation s = (ρhga/2) where s is the surface. Fill a glass, cup, or small bowl with tap water. in this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. In order to do this, you will determine the number of. Water, soapy water, or rubbing alcohol.
From www.researchgate.net
Schematic illustration of standard methods of surface tension Measuring Surface Tension Of Water With A Penny Fill the medicine dropper with water. place your penny on a flat, level surface that can get a little wet, like a kitchen counter. water has very unique properties, and this experiment puts them to the test!. in this portion of the lab you will determine which liquid has the highest surface tension: 15k views 14 years. Measuring Surface Tension Of Water With A Penny.
From www.wikihow.com
3 Ways to Measure Surface Tension wikiHow Measuring Surface Tension Of Water With A Penny Fill a glass, cup, or small bowl with tap water. You can fit many more drops of water on top of a penny. In order to do this, you will determine the number of. Fill the medicine dropper with water. in this portion of the lab you will determine which liquid has the highest surface tension: 15k views 14. Measuring Surface Tension Of Water With A Penny.
From www.pinterest.co.uk
Surface Tension Water Experiment Water Drops On A Penny Science Measuring Surface Tension Of Water With A Penny Water, soapy water, or rubbing alcohol. Fill the medicine dropper with water. place your penny on a flat, level surface that can get a little wet, like a kitchen counter. 15k views 14 years ago. In order to do this, you will determine the number of. in this activity you will see how soap decreases the surface tension. Measuring Surface Tension Of Water With A Penny.
From www.sciencebuddies.org
Measuring Surface Tension of Water with a Penny Measuring Surface Tension Of Water With A Penny Fill a glass, cup, or small bowl with tap water. water has very unique properties, and this experiment puts them to the test!. You can fit many more drops of water on top of a penny. Water, soapy water, or rubbing alcohol. attraction of the water molecules holds the surface of the water together until the amount of. Measuring Surface Tension Of Water With A Penny.
From www.sciencebuddies.org
Measuring Surface Tension of Water with a Penny Science Project Measuring Surface Tension Of Water With A Penny place your penny on a flat, level surface that can get a little wet, like a kitchen counter. in this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. You can fit many more drops of water on top of a penny. water has very. Measuring Surface Tension Of Water With A Penny.
From exomhdjxh.blob.core.windows.net
How To Measure Surface Tension Of A Liquid at Dina Moose blog Measuring Surface Tension Of Water With A Penny attraction of the water molecules holds the surface of the water together until the amount of water is too great and spills over the. surface tension is given by the equation s = (ρhga/2) where s is the surface. Water, soapy water, or rubbing alcohol. You can fit many more drops of water on top of a penny.. Measuring Surface Tension Of Water With A Penny.
From www.youtube.com
Surface Tension Experiment Water and Our World The Good and the Measuring Surface Tension Of Water With A Penny Fill the medicine dropper with water. attraction of the water molecules holds the surface of the water together until the amount of water is too great and spills over the. Water, soapy water, or rubbing alcohol. water has very unique properties, and this experiment puts them to the test!. In order to do this, you will determine the. Measuring Surface Tension Of Water With A Penny.
From clearsolutionsusa.com
How to Measure Surface Tension Clear Solutions USA Measuring Surface Tension Of Water With A Penny Fill the medicine dropper with water. in this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. attraction of the water molecules holds the surface of the water together until the amount of water is too great and spills over the. place your penny on. Measuring Surface Tension Of Water With A Penny.
From www.youtube.com
Water's surface tension physics experiment YouTube Measuring Surface Tension Of Water With A Penny You can fit many more drops of water on top of a penny. in this portion of the lab you will determine which liquid has the highest surface tension: Water, soapy water, or rubbing alcohol. Fill a glass, cup, or small bowl with tap water. Fill the medicine dropper with water. surface tension is given by the equation. Measuring Surface Tension Of Water With A Penny.
From lambdasys.com
Physics Experiment LEMI30 Apparatus of Measuring Liquid Surface Measuring Surface Tension Of Water With A Penny place your penny on a flat, level surface that can get a little wet, like a kitchen counter. water has very unique properties, and this experiment puts them to the test!. Water, soapy water, or rubbing alcohol. Fill a glass, cup, or small bowl with tap water. You can fit many more drops of water on top of. Measuring Surface Tension Of Water With A Penny.
From thesciencekiddo.blogspot.com
Surface Tension Drops of Water on a Coin + Free Printable The Measuring Surface Tension Of Water With A Penny In order to do this, you will determine the number of. surface tension is given by the equation s = (ρhga/2) where s is the surface. 15k views 14 years ago. in this portion of the lab you will determine which liquid has the highest surface tension: place your penny on a flat, level surface that can. Measuring Surface Tension Of Water With A Penny.
From www.pinterest.com
Question Can You Measure Surface Tension of Water With a Penny Measuring Surface Tension Of Water With A Penny place your penny on a flat, level surface that can get a little wet, like a kitchen counter. in this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. Fill a glass, cup, or small bowl with tap water. You can fit many more drops of. Measuring Surface Tension Of Water With A Penny.
From www.rookieparenting.com
How Many Drops of Water Can Fit on a Penny Surface Tension Experiment Measuring Surface Tension Of Water With A Penny In order to do this, you will determine the number of. in this activity you will see how soap decreases the surface tension of water by putting water droplets on top of a penny. water has very unique properties, and this experiment puts them to the test!. in this portion of the lab you will determine which. Measuring Surface Tension Of Water With A Penny.
From ar.inspiredpencil.com
Surface Tension Of Water On A Penny Measuring Surface Tension Of Water With A Penny attraction of the water molecules holds the surface of the water together until the amount of water is too great and spills over the. You can fit many more drops of water on top of a penny. Water, soapy water, or rubbing alcohol. surface tension is given by the equation s = (ρhga/2) where s is the surface.. Measuring Surface Tension Of Water With A Penny.
From deceptivelyeducational.blogspot.com
Relentlessly Fun, Deceptively Educational How many drops of water fit Measuring Surface Tension Of Water With A Penny 15k views 14 years ago. water has very unique properties, and this experiment puts them to the test!. Fill a glass, cup, or small bowl with tap water. You can fit many more drops of water on top of a penny. in this activity you will see how soap decreases the surface tension of water by putting water. Measuring Surface Tension Of Water With A Penny.
From www.youtube.com
Surface Tension Super Ability Of Liquid Water on Penny Experiment Measuring Surface Tension Of Water With A Penny surface tension is given by the equation s = (ρhga/2) where s is the surface. Fill a glass, cup, or small bowl with tap water. Fill the medicine dropper with water. In order to do this, you will determine the number of. 15k views 14 years ago. Water, soapy water, or rubbing alcohol. place your penny on a. Measuring Surface Tension Of Water With A Penny.
From easyscienceforkids.com
Comparing Surface Tension of Liquids with Pennies Physics Experiment Measuring Surface Tension Of Water With A Penny place your penny on a flat, level surface that can get a little wet, like a kitchen counter. In order to do this, you will determine the number of. 15k views 14 years ago. Water, soapy water, or rubbing alcohol. surface tension is given by the equation s = (ρhga/2) where s is the surface. You can fit. Measuring Surface Tension Of Water With A Penny.
From ar.inspiredpencil.com
Surface Tension Penny Measuring Surface Tension Of Water With A Penny In order to do this, you will determine the number of. water has very unique properties, and this experiment puts them to the test!. Fill the medicine dropper with water. 15k views 14 years ago. attraction of the water molecules holds the surface of the water together until the amount of water is too great and spills over. Measuring Surface Tension Of Water With A Penny.